Hydrogen is an energy carrier of high density that offers an alternative to nonrenewable and polluting petroleum-based fuels for transportation. A multidisciplinary team of researchers at Penn State, Cornell and NREL demonstrate an effective screening procedure to maximize the success rate of materials discovery for the solar generation of hydrogen fuels. Image: Penn State
Computers help researchers find materials to turn solar power into hydrogen
Posted on June 16, 2021UNIVERSITY PARK, Pa. — Using solar energy to inexpensively harvest hydrogen from water could help replace carbon-based fuel sources and shrink the world’s carbon footprint. However, finding materials that could boost hydrogen production so that it could compete economically with carbon-based fuels has been, as yet, an insurmountable challenge.
In a study, a Penn State-led team of researchers report they have taken a step toward overcoming the challenge of inexpensive hydrogen production by using supercomputers to find materials that could help accelerate hydrogen separation when water is exposed to light, a process called photocatalysis.
Finding materials that boost hydrogen production is a step toward competing economically with carbon-based fuels
Both electricity and solar energy can be used to separate hydrogen from water, which is made up of two hydrogen atoms and an oxygen atom, according to Ismaila Dabo, associate professor of materials science and engineering. Institute for Computational and Data Sciences (ICDS) affiliate and co-funded faculty member of the Institutes of Energy and the Environment. Using sunlight to generate electricity to create hydrogen — or electrolysis –, which, in turn, would likely be converted back into electricity may not be technically advantageous or economically effective. While using solar energy directly to produce hydrogen from water — or photocatalysis — avoids that extra step, researchers have yet to be able to use direct solar hydrogen conversion in a way that would compete with carbon-based fuels, such as gasoline.
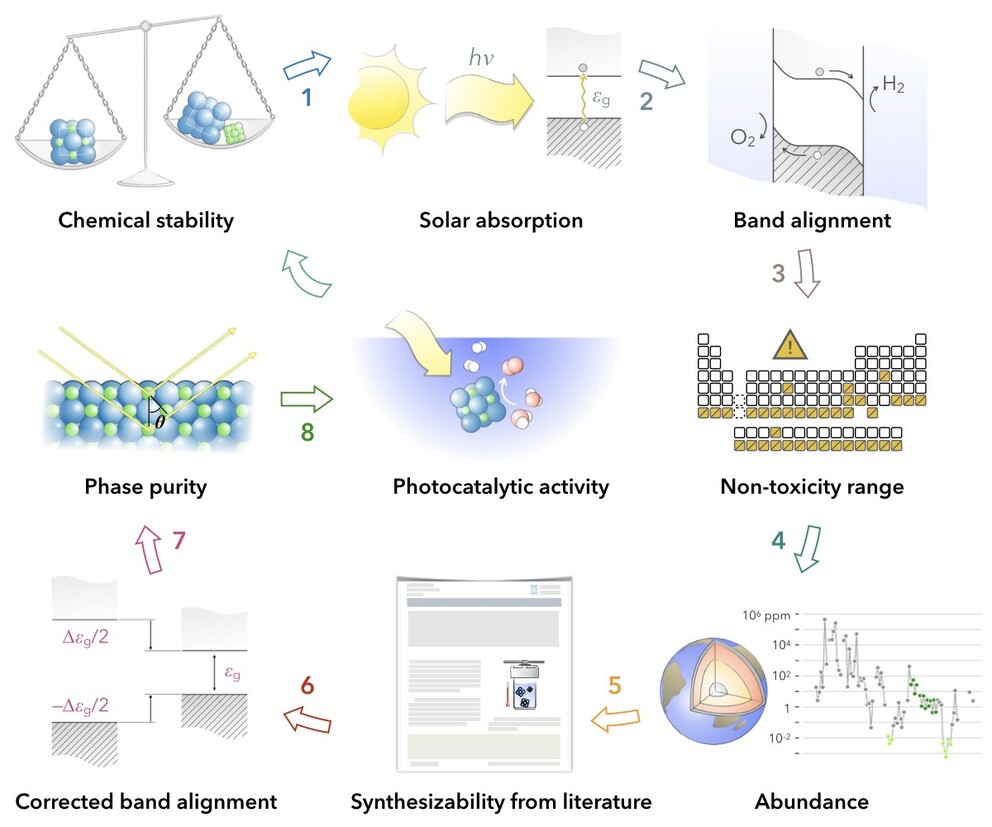
The researchers, who report their findings in Energy and Environmental Science, used a type of computational approach called high-throughput materials screening to narrow a list of more than 70,000 different compounds down to six promising candidates for those photocatalysts, which, when added to water, can enable the solar hydrogen production process, said Dabo.
They examined the compounds listed in the Materials Project database, an online open-access repository of known and predicted materials. The team developed an algorithm to identify materials with properties that would make them suitable photocatalysts for the hydrogen production process. For example, the researchers investigated the ideal energy range — or the band gap — for the materials to absorb sunlight. Working closely with Héctor Abruña, professor of chemistry at Cornell; Venkatraman Gopalan, professor of materials science and engineering at Penn State; and Raymond Schaak, professor of chemistry at Penn State; they also looked at materials that could effectively dissociate water, as well as materials that offered good chemical stability.
“We believe the integrated computational-experimental workflow that we have developed can considerably accelerate the discovery of efficient photocatalysts,” said Yihuang Xiong, graduate research assistant and co-first author of the paper. “We hope that, by doing so, we will be able to reduce the cost of hydrogen production.”
Dabo added the team focused on oxides — chemical compounds made up of at least one oxygen atom — because they can be synthesized in a reasonable amount of time using standard processes. The work required collaborations from across disciplines, which served as a learning experience for the research team.
“I found it very rewarding to have worked on such a collaborative project,” said Nicole Kirchner-Hall, doctoral student and co-author of the paper. “As a graduate student specializing in computational material science, I was able to predict possible photocatalytic materials using calculations and work with experimental collaborators here at Penn State and other institutions to co-validate our computational predictions.”
Other researchers have previously conducted an economic analysis on several options of using solar energy to produce electricity and determined that solar could drop the price of hydrogen production to compete with gasoline, said Dabo.
“Their essential conclusion was that if you were able to develop this technology, you could produce hydrogen at the cost of $1.60 to $3.20 per equivalent gallon of gasoline,” said Dabo. “So, compare that to gasoline, which is around $3 a gallon, if this works, you could pay as low as $1.60 for about the same amount of energy as a gallon of gas in the ideal case scenario.”
He added that if a catalyst can help boost solar hydrogen production, this could lead to a hydrogen price that is competitive with gasoline.
The team relied on Penn State ICDS’s Roar supercomputer for the computations. According to Dabo, computers represent an important tool in speeding up the process to find the right materials to be used in specific processes. This computationally driven, data-intensive method could represent a revolution in efficiency over the painstaking trial-and-error approach.
“When Thomas Edison wanted to find materials for the light bulb, he looked at just about every material under the sun until he found the right material for the light bulb,” said Dabo. “Here we’re trying to do the same thing, but in a way to use computers to accelerate that process.”
He added that computers will not replace experimentation.
“Computers can make the recommendations as to what materials will be the most promising and then you still need to do the experimental study,” said Dabo.
Dabo said he expects the power of computers will streamline the process of finding the best candidates and dramatically cut the time it takes to design materials in the lab to bring them to market to address needs.
The researchers evaluated machine learning algorithms to make suggestions for chemicals that could be synthesized and used as catalysts in solar hydrogen production. Based on this preliminary investigation, they suggest future work may focus on developing machine learning models to improve the chemical screening process.
Dabo also added that they may look at chemical compounds outside of oxides to determine if they might serve as catalysts for solar hydrogen production.
“So far, we did one cycle of this process on oxides — essentially rusted metals — but there are a lot of compounds that could be made that aren’t based on oxygen,” said Dabo. “For example, there are compounds based on nitrogen or sulfur, that we could explore.”
The National Science Foundation (NSF) and the HydroGEN Advanced Water Splitting Materials Consortium of the U.S. Department of Energy (DOE) supported this work.
The team also included Quinn T. Campbell, postdoctoral scholar at Sandia National Laboratories; Julian Fanghanel, graduate student in materials science and engineering at Penn State; Catherine K. Badding, undergraduate student in chemistry at Cornell and DOE Science Undergraduate Laboratory Internship Fellow (now graduate student at MIT); Huaiyu Wang, graduate student in materials science and engineering at Penn State; Monica J. Theibault, graduate student in chemistry at Cornell; Iurii Timrov, postdoctoral scholar at École Polytechnique Fédérale de Lausanne, Switzerland; Jared S. Mondschein, graduate student in chemistry at Penn State; Kriti Seth, graduate student in chemistry at Penn State; Rebecca Katz, graduate student in chemistry at Penn State; Andrés Molina Villarino, graduate student in chemistry at Cornell; Betül Pamuk, postdoctoral scholar in physics at Cornell; Megan E. Penrod, undergraduate student in materials science and engineering at Penn State (now graduate student at University of Florida); Mohammed M. Khan, undergraduate student in materials science and engineering at Penn State (now graduate student at KAUST); Tiffany Rivera, NSF Research Experiences for Undergraduates Fellow; Nathan C. Smith, NSF Research Experiences for Undergraduates Fellow; Xavier Quintana, NSF Research Experiences for Undergraduates Fellow; Paul Orbe, NSF Research Experiences for Teachers Fellow; Craig J. Fennie, professor of physics at Cornell; Senorpe Asem-Hiablie, courtesy assistant research professor at the Penn State Institutes of Energy and the Environment; James L. Young, researcher at the National Renewable Energy Laboratory; Todd G. Deutsch, researcher at the National Renewable Energy Laboratory; and Matteo Cococcioni, professor of physics at University of Pavia, Italy.
Share
Related Posts
- Designed to Adapt: Living materials are the future of sustainable building
- $500,000 grant funds creation of institute to advance AI for materials science
- Smart materials could pose solution for big-data bottleneck in future cities
- AI behind deepfakes may power materials design innovations, scientists say
